What is d-d transition ? (From d & f block elements )
Answer:- The electronic transition between two energy levels of d orbital is called d-d transition. Movement of electron from one d orbital to other d orbital having different energy.
Where does d-d transition occur ?
Ans:- d-d transition is found in complex compounds ( or Coordination compounds ). Complex compound is made up of two entities – one is central metal atom / ion and other is Ligand (electron donor molecule or atom or ion )
Why d-d transition occur in place of simple electronic transition occurs between different shells?
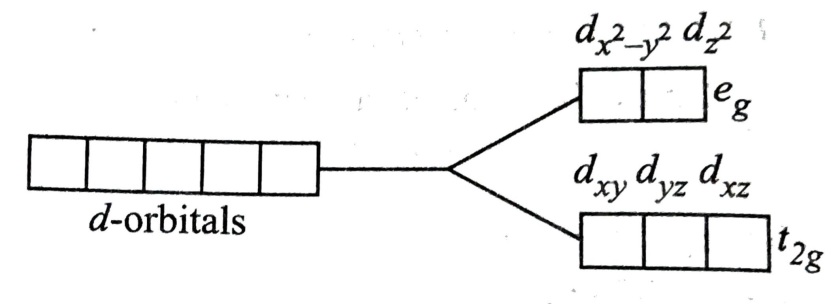
Answer:- In coordination / complex compounds d subshell containing five d orbitals split into two parts (t2g and eg ) under influence of ligand field.
In octahedral complex / coordination compounds d subshell is splited into two energy level: one is of lower energy called t2g energy level containing three d orbital dxy, dyx and dzx and other is of higher energy level called eg energy level containing two d orbitals d x2-y2 and dz2
Why complex / coordination compounds are coloured ?
In coordination / complex compound, When electron present in one of t2g orbital absorb fixed amount of energy ( absorb particular frequency or wavelength of white light ) and moves to one of eg orbital than complementary light is reflected which we see through our eyes.
e.g. CuSO4.5H2O is blue in coloured,
Sodium chromate is yellow in coloured.
Sodium dichromate is Orange in coloured
[Cu (H2O)4]2+ absorb at 600nm means Red colour and complementary colour we see is Green.