The reaction in which the carbonyl compound (having no alpha hydrogen) is treated with base (NaOH or KOH) to produce alcohol and Carboxylate ion is called Cannizzaro’s reaction.
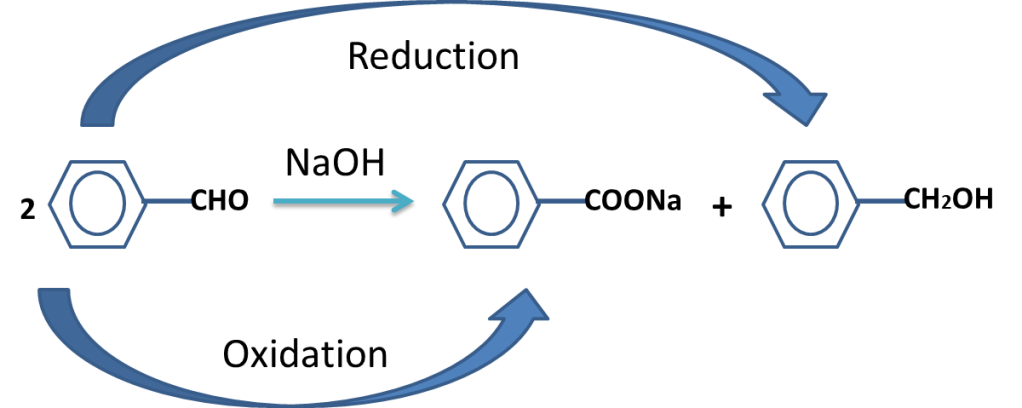
The cannizzro,s reaction is given by the aldehydes which has no alpha hydrogen.
The aldehyde oxidizes to carboxylate ion and reduced to alcohol.
Cannizzaro’s reaction is disproportionation reaction.
The reaction in which same molecule oxidizes and reduced simultaneously is called disproportionation reaction..
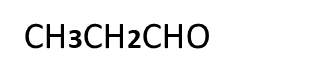
Question:- Which compounds gives Cannizzaro’s reaction out of the following compounds and why?
A
B


C.
D.
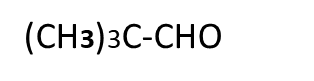
Answer:-Compounds A and C gives Cannizzaro’s reaction because they have absence of alpha hydrogen atom.