Question:-Explain the paramagnetic nature of oxygen and calculate bond order by drawing molecular orbital orbital diagram and molecular orbital electronic configuration.
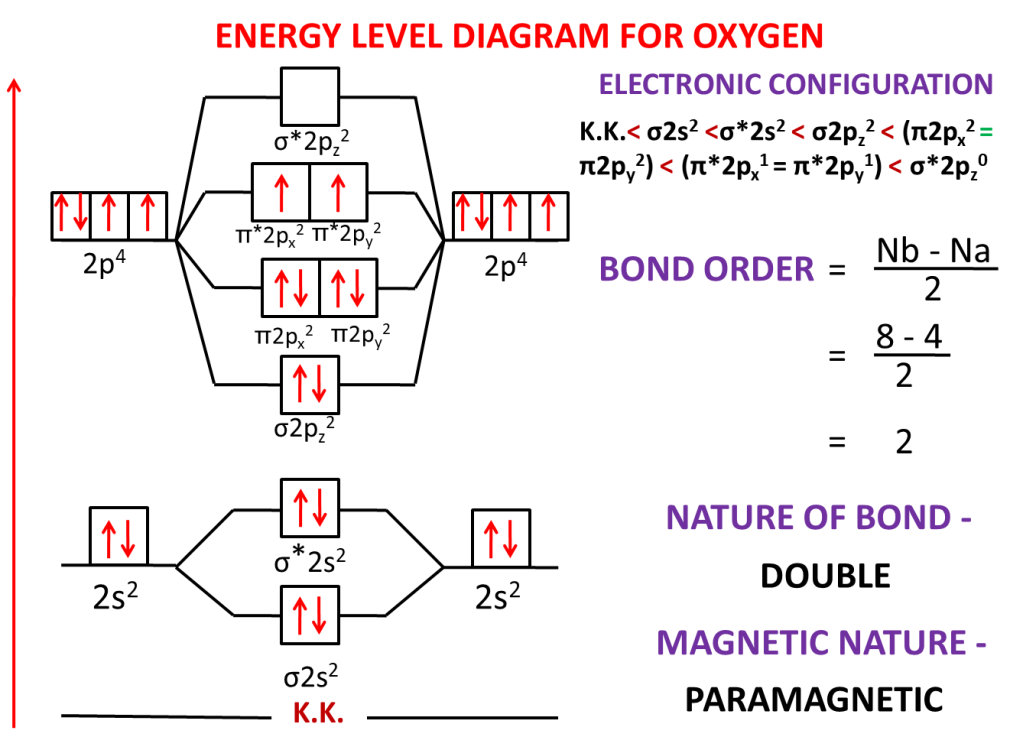
Question:-Explain the paramagnetic nature of oxygen and calculate bond order by drawing molecular orbital orbital diagram and molecular orbital electronic configuration.

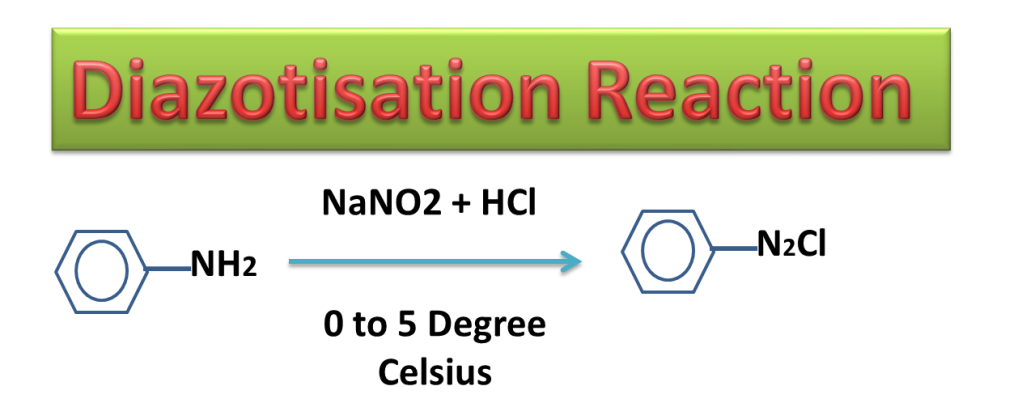
In diazotization Reaction, The aniline is treated with nitrous acid ( a mixture of sodium nitrite and hydrochloric acid) at temperature 00 to 50 Celsius ( 273 K to 278 K ) to produce diazonium chloride.
Nitrous acid is produce in the reaction mixture by treating NaNO2 with HCl
NaNO2 + HCl —> HNO2 + NaCl
Diazonium salt is highly unstable and can explode at little higher temperature therefore it is prepared at lower temperature (00 to 50 Celsius).
Diazonium salt can not be separated and product mixture is used for further reaction because diazonium salt is highly unstable and explode on distillation (on heating).
Diazonium salt is very much important in organic synthesis as for preparation of large number of organic compounds, it is precursor (Reactant).
Answers are given in the end-
1.Amines are basic in nature . Why?
2.Arrange the following amines in decreasing order of their basic strength. Give reason also.
(CH3)3N > (CH3)2NH > CH3NH2 > NH3 In aqueous phase
3. Arrange the following amines in decreasing order of their basic strength. Give reason also.
C2H5NH2 > (C2H5)2NH > (C2H5)3N > NH3 In aqueous phase
4. Arrange the following amines in decreasing order of their basic strength. Give reason also.
(CH3)2NH > (CH3)3N > CH3NH2 > NH3 in gaseous phase
5.Arrange the following amines in decreasing order of their basic strength. Give reason also.

6. Methanamine is less basic than aniline. Why?
7.Anilene does not gives friedal craft,s reaction.Why?
8.Why does amine group is protected by acetylating it?
9. Explain briefly
(a) Carbylamine reaction( Isocyanide test)
10. pKb value of ethylamine is lower than benzylamine
Answer
1.Basic nature of amine is attributed to presence of lone pair of electron on nitrogen atom of amine group which can be donated.
2. (CH3)2NH > CH3NH2 > (CH3)3N > NH3 (This order is combine effect of three factors.In aqueous phase, inductive effect, solvation effect and steric hindrance also play important roles)
3.(C2H5)2NH > (C2H5)3N > C2H5NH2 > NH3 (This order is combine effect of three factors.In aqueous phase, inductive effect, solvation effect and steric hindrance also play important roles)
4. (CH3)3N > (CH3)2NH > CH3NH2 > NH3 ( Due to Steric hinderance increases from primary to tertiary amine which hinder to donate lone pair of electron.)
5.

Methyl group, having +I effect increases the electron density on Nitrogen of amine whereas electron withdrawing(-I and –M effect) nitro group decreases electron density on nitrogen of benzene ring.
6.Due to +I effect of methyl group electron density increases in N of amine group whereas electron density decreases due to involvement of lone pair of electrons in resonance.
7. Because basic group amine (-NH2) reacts with friedal craft,s reagents which is acidic in nature and nitrogen bears +ive charge. This +vely charged nitrogen deactivate to the benzene ring due to its elecrtron withdrawing effect.
8. Amine group is protected by acetylating ( addition of -COCH3) it therefore it does not react with friedal craft,s reagens and able to perform friedal craft reaction.
9.When primary amine is treated with chloroform (CHCl3) in presence of base (KOH) yields isocyanide having offensive smell.
R-NH2 + CHCl3 +3 KOH –> R-NC + 3KCl + H2O
10.( pKb value lower mean basic strength is higher) Due to +I effect of ethyl group electron density increases in N of amine group, therefore ethamine is more basic whereas aniline is less basic as electron density decreases due to involvement of lone pair of electrons in resonance.
Aldehydes Ketones and Ethers:- Important and conceptual questions
Answers are given in the end-
1. Why does carbonyl compounds give Nucleophylic reaction?
2. Arrange the following carbonyl compounds in increasing order of their reactivities towards nucleophylic addition reaction.
CH3CHO, HCHO, CH3COCH3
3.Which of the following compounds give iodoform reaction and why?
CH3CHO, HCHO, CH3CH2COCH2CH3
4 Which of the following compounds give cannizaro,s reaction and why?
CH3CHO, HCHO, CH3CH2COCH2CH3
5. Which of the following compounds give aldol condensation reaction and why?
CH3CHO, HCHO, CH3CH2COCH2CH3
6. How many compounds are formed in the cross aldol condensation of
A) CH3CHO, CH3COCH3
B) Ph-CHO and CH3CHO
C) Ph-CO-Ph and CH3CHO
D) CH3COCH3 and CH3CH2COCH2CH3
E) Ph-CHO and Ph-CO-Ph
7.What are the disproportion reaction. Give example.
Answer-
1. Nucleophylic reaction reaction occure due to presence of partial positive charge on carbonyl carbon and addition reaction occure due to presence of double bond.
2. CH3COCH3 < CH3CHO < HCHO
3. CH3CHO, The compounds have three alpha hydrogen atoms to carbonyl groups gives iodoform reaction.
4. HCHO, The compounds have no any alpha hydrogen atoms to carbonyl groups gives cannizaro,s reaction.
5. CH3CHO and CH3CH2COCH2CH3 The compounds have atleast one alpha hydrogen atoms to carbonyl groups gives aldol condensation reaction.
6. A) Four aldol products
B) Two aldol products
C)Two aldol products
D) Four aldol products
E) No aldol products
7. The reaction in which same species is oxidized and reduced simultaneously.
Example is cannizaro,s reaction in which carbonyl compounds having no alpha hydrogen is oxidized in carboxylate ion and reduced to alcohol.
Ph-CHO + KOH à Ph-COO– + Ph-CH2-OH