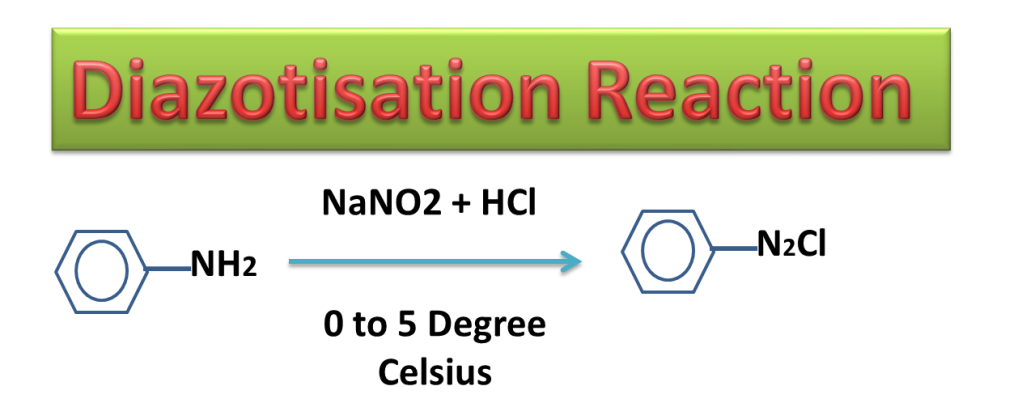
In diazotization Reaction, The aniline is treated with nitrous acid ( a mixture of sodium nitrite and hydrochloric acid) at temperature 00 to 50 Celsius ( 273 K to 278 K ) to produce diazonium chloride.
Nitrous acid is produce in the reaction mixture by treating NaNO2 with HCl
NaNO2 + HCl —> HNO2 + NaCl
Diazonium salt is highly unstable and can explode at little higher temperature therefore it is prepared at lower temperature (00 to 50 Celsius).
Diazonium salt can not be separated and product mixture is used for further reaction because diazonium salt is highly unstable and explode on distillation (on heating).
Diazonium salt is very much important in organic synthesis as for preparation of large number of organic compounds, it is precursor (Reactant).
