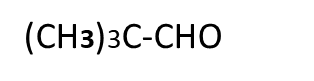Aldehydes Ketones and Ethers:- Important and conceptual questions
Answers are given in the end-
1. Why does carbonyl compounds give Nucleophylic reaction?
2. Arrange the following carbonyl compounds in increasing order of their reactivities towards nucleophylic addition reaction.
CH3CHO, HCHO, CH3COCH3
3.Which of the following compounds give iodoform reaction and why?
CH3CHO, HCHO, CH3CH2COCH2CH3
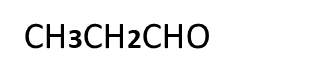
4 Which of the following compounds give cannizaro,s reaction and why?
CH3CHO, HCHO, CH3CH2COCH2CH3
5. Which of the following compounds give aldol condensation reaction and why?
CH3CHO, HCHO, CH3CH2COCH2CH3
6. How many compounds are formed in the cross aldol condensation of
A) CH3CHO, CH3COCH3
B) Ph-CHO and CH3CHO
C) Ph-CO-Ph and CH3CHO
D) CH3COCH3 and CH3CH2COCH2CH3
E) Ph-CHO and Ph-CO-Ph
7.What are the disproportion reaction. Give example.
Answer-
1. Nucleophylic reaction reaction occure due to presence of partial positive charge on carbonyl carbon and addition reaction occure due to presence of double bond.
2. CH3COCH3 < CH3CHO < HCHO
3. CH3CHO, The compounds have three alpha hydrogen atoms to carbonyl groups gives iodoform reaction.
4. HCHO, The compounds have no any alpha hydrogen atoms to carbonyl groups gives cannizaro,s reaction.
5. CH3CHO and CH3CH2COCH2CH3 The compounds have atleast one alpha hydrogen atoms to carbonyl groups gives aldol condensation reaction.
6. A) Four aldol products
B) Two aldol products
C)Two aldol products
D) Four aldol products
E) No aldol products
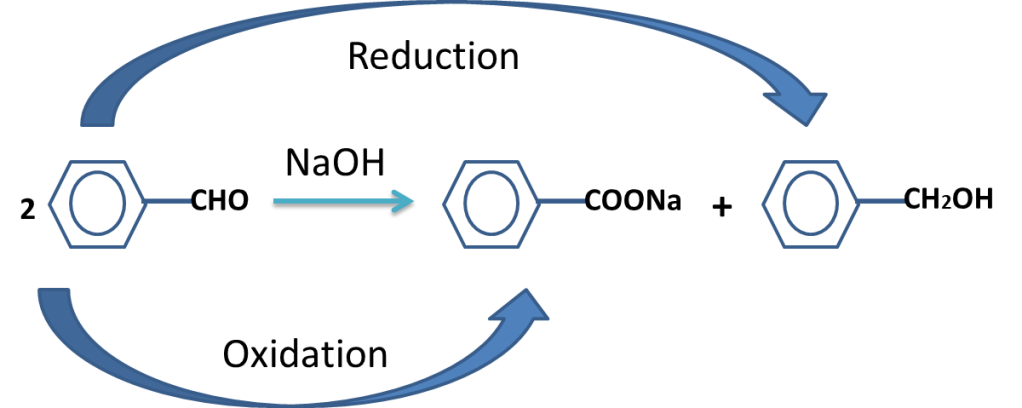
7. The reaction in which same species is oxidized and reduced simultaneously.
Example is cannizaro,s reaction in which carbonyl compounds having no alpha hydrogen is oxidized in carboxylate ion and reduced to alcohol.
Ph-CHO + KOH à Ph-COO– + Ph-CH2-OH