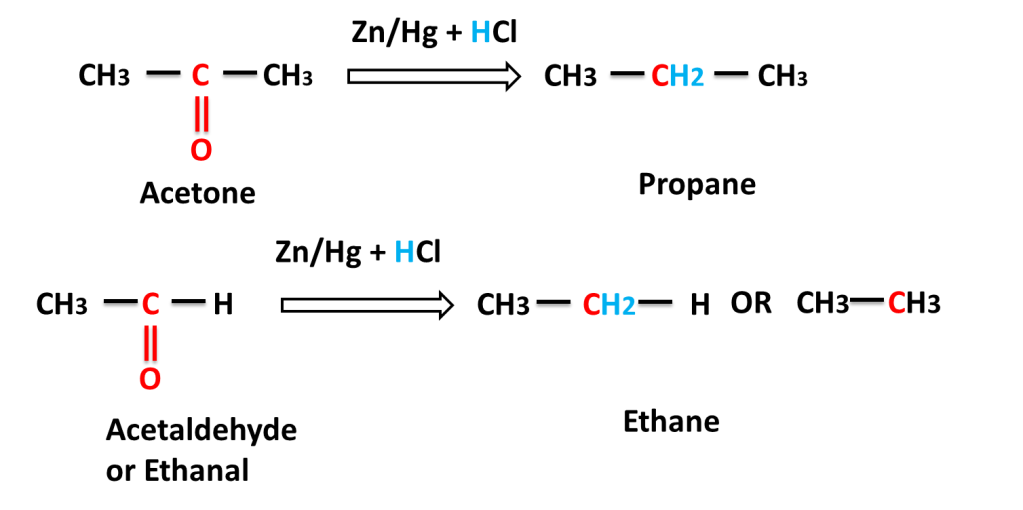
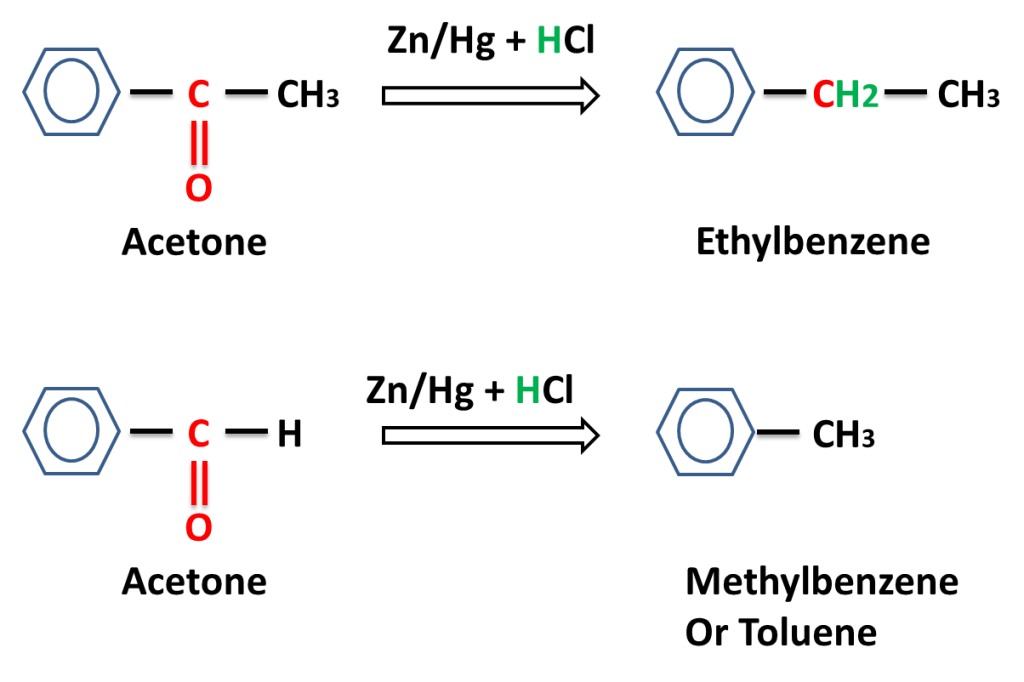
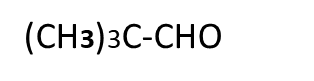The reaction in which carbonyl group (C=O) is reduced into (-CH2-) group on reacting with a mixture of zinc and hydrochloric acid is called Clemmensen,s Reduction.
General reaction:-
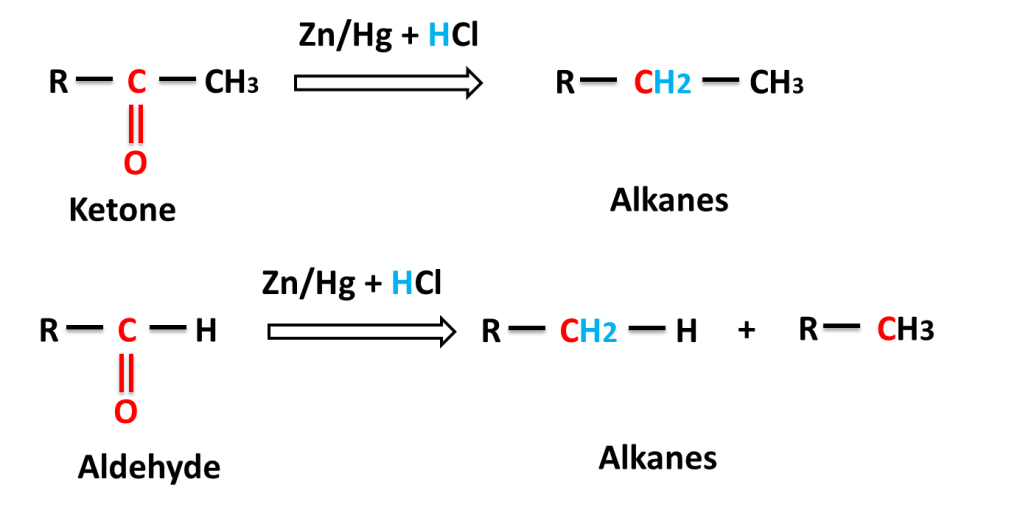
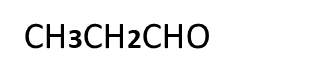
Reaction of aliphatic aldehyde and ketones-
Reaction of aromatic aldehyde and ketones-
Question:- Why did a mixture of Zn+HCl is used for reduction ?
Answer:- Zn reaction reacts with HCl to produce H2 gas which reduces carbonyl (C=O) to alkane (-CH2-).
Question:- Why does amalgam of Zinc (Zn/Hg) use in spite of only Zn ?
Answer:- Zn reacts with HCl to produce H2 very fast that move out fast from reaction mixture and carbonyl compound has very little time to react and most of hydrogen moves out of reaction mixture without reacting whereas in form of amalgam, Zn reacts slowly to produce hydrogen slowly, which can react with carbonyl compound. because only those Zn atoms reacts with HCl which are present on the surface of amalgam .
Amalgam: The solution of metal in mercury is called amalgam. Metal dissolve in mercury like salt dissolve in water.